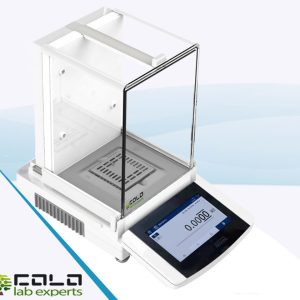
MEUSE3 Micro Balance
Note: Supplied with glass draft shield, stainless-steel pan Ø30 mm, AC adapter (110–240 V), and user manual (EN).
Highlights: 0.001 mg readability, ±0.002 mg repeatability, ±0.005 mg linearity, automatic internal calibration, GLP/GMP printout.
Key features
- Automatic internal calibration (time/temperature triggers) + support for external mass verification.
- 7″ high-resolution full-touch UI with quick access to applications and logs.
- 0.001 mg readability with ±0.002 mg repeatability and ±0.005 mg linearity.
- GLP/GMP compliant data export; RS-232C interface for LIMS/printers.
- Draft-shield chamber optimized for micro-weighing stability; anti-vibration friendly.
- Auto/Manual temperature compensation, auto-hold, stability indicator.
- Universal supply AC 110–240 V.
Specifications
| Parameter | MEUSE3 |
|---|---|
| Capacity | 3 g |
| Readability | 1 µg (0.001 mg) |
| Repeatability | ±0.002 mg |
| Linearity | ±0.005 mg |
| Pan Size | Ø 30 mm |
| Display | 7″ high-resolution full-touch screen |
| Calibration | Automatic internal; external mass check supported |
| Output Interface | RS-232C |
| Power Supply | AC 110–240 V |
| Net / Gross Weight | 6.8 kg / 9.0 kg |
Included
- MEUSE3 micro balance unit
- Glass draft shield assembly
- Stainless-steel pan Ø30 mm
- AC adapter (110–240 V)
- User manual (EN)
Recommended accessories
- Anti-vibration table
- Certified E2 class mass set (for external checks)
- RS-232C printer / LIMS cable
- Automatic door technology
- Ionizer (electrostatic removal)
- Second screen
- Pharmaceutical software package
- External draft shield
- Gesture sensor
- Portable micro printer
Calibration & good practice
- Enable auto internal calibration (time/temperature) and verify with E2 masses per SOP.
- Warm-up 30–60 min; use anti-vibration support; control drafts and temperature gradients.
- Handle micro-masses with tweezers and gloves; use weighing boats where applicable.
Performance & technology
- High-stability micro weighing cell with low-noise electronics and thermal design for fast, repeatable readings.
- 1 µg readability, typical ±0.002 mg repeatability and ±0.005 mg linearity in routine use.
- Fast stabilization thanks to optimized draft-shield airflow and refined digital filtering.
Calibration & verification
- Automatic internal calibration (time / temperature triggers) maintains accuracy over the day.
- External verification supported with certified E2 weights according to your SOP.
- Traceable printouts with date/time, ID and result lines for GLP/GMP records.
Compliance & data integrity
- GLP/GMP compliant printout via RS-232C; easy integration with LIMS/printers.
- Optional (Pharma software package): user roles and access control, audit trail with timestamps, electronic signatures for weighing reports, and controls aligned with ALCOA principles and 21 CFR Part 11 expectations.
Automation & convenience
- Optional: Motorized draft-shield doors with position memory for non-contact operation.
- Optional: Built-in ionizer to automatically neutralize static on vessels and samples.
- 7″ full-touch interface for fast navigation, apps and logs; stability indicator, auto-hold, temperature compensation.
- RS-232C interface for printers/LIMS; universal power supply AC 110–240 V.